Revisiting the Need for MDI Common Canister Protocols During the COVID-19 Pandemic
Patients infected with the coronavirus (COVID-19 virus) often require inhaled bronchodilator medications (e.g., albuterol, levalbuterol). Because nebulizer therapy with bronchodilators for presumptive or confirmed COVID-19 patients may not be safe due to the generation of aerosols, which increases the risk that respiratory droplets will remain in the air and spread the virus, delivery of these drugs via metered-dose inhalers (MDIs) is preferred. As a result, use of these inhalers has skyrocketed during the pandemic and there is concern about inhaler drug shortages. Supply chain disruptions are already being experienced in some areas, leaving some hospitals with just a few days supply. MDI canisters usually contain enough medication to last 2-4 weeks, while patients are often hospitalized for shorter periods, frequently leading to drug waste. As a result, hospitals are considering the best way to conserve MDI supplies.
Some organizations are asking patients to bring in a prescribed MDI to use throughout their hospitalization. Or, when the pharmacy dispenses an MDI for a specific patient, they are immediately labeling it for home use so the MDI can be sent home with the patient at discharge. Others are considering, or have implemented, a common MDI canister protocol as another way to address possible shortages. ISMP has been asked about our position on the latter topic.
In 2009, we published an article about the risks and benefits of using a common MDI canister, a patient-specific spacer, and a disinfection procedure between patients to administer doses from the same MDI to multiple patients. At that time, common canister policies were being utilized by respiratory therapists and nurses who disinfected the MDI after administering each dose, and then reused it for a different patient’s dose, primarily as a cost-savings measure, not for conserving inhalers to help alleviate drug shortages. The common canister protocols called for disinfecting the mouthpiece with an alcohol prep pad before inserting it into a patient-specific spacer with a one-way valve (Figure 1), administering the medication, and then disinfecting the mouthpiece after use. In our 2009 newsletter article, we cited studies that showed varying levels of bacterial contamination on disinfected mouthpieces, from no growth up to 5% with contamination.
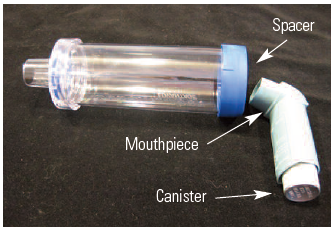 Figure 1. MDI with spacer device.
Figure 1. MDI with spacer device.
Deciding whether to implement a common MDI canister protocol during the COVID-19 pandemic requires thoughtful analysis and deliberation. Importantly, even in 2009, we pointed out three critical risk factors that still exist today: 1) Using a common canister may not be appropriate for patients on isolation precautions or for immunocompromised patients; 2) The methods used for disinfecting the mouthpiece (using alcohol wipes) in 2009 were aimed at preventing bacterial contamination, which would not be considered adequate for the COVID-19 virus; and 3) Individual noncompliance with always following the mouthpiece disinfection protocol is a concern. Safer protocols used today call for dual cleaning between nursing or respiratory therapy and pharmacy to reduce the risk of individual staff noncompliance with disinfecting the mouthpiece. In addition, segregation of disinfected MDIs to delay their reuse and/or sterilization procedures are used in some hospitals before an MDI can subsequently be reused for another patient.
Unfortunately, ISMP is not in a position to develop timely guidelines for a common canister protocol or to endorse individual hospital protocols currently available or being considered. However, we have alerted the Centers for Disease Control and Prevention (CDC), the Association for Professionals in Infection Control and Epidemiology (APIC), and the American Association for Respiratory Care (AARC) about the need for clear guidance on this subject. Meanwhile, we recognize that many hospitals are facing a shortage of MDI bronchodilators.
Until such guidance occurs, we have provided a summary of the important components of an MDI common canister protocol recently developed by Spectrum Health Butterworth Hospital in Grand Rapids, MI, in response to the COVID-19 pandemic and the impending drug shortage (Table 1). We greatly appreciate the hospital’s willingness to explore this topic with ISMP and share key components of its emerging protocol, which can be used by other hospitals to develop or review a similar protocol. Instead of disinfecting the MDI for reuse between doses, this hospital’s policy calls for a dual disinfection process and reuse only after the MDI has been discontinued and/or the patient has been discharged.
Table 1. Components of a common MDI canister protocol from Spectrum Health Butterworth Hospital
| Task |
Component |
| Dispensing |
New MDI is dispensed from the pharmacy or via an automated dispensing cabinet |
| Nurses or pharmacy staff label the MDI for the specific patient when dispensed |
| Initiation |
All patients prescribed an MDI medication receive a patient-specific spacer device |
| Nurses or respiratory therapists (RTs) label the spacer device for the specific patient |
| Administration |
Nurses or RTs attach the patient-specific MDI to the patient-specific spacer to deliver each dose |
| Storage |
Labeled patient-specific spacer is stored in a plastic bag at the bedside between uses |
| Labeled patient-specific MDI is stored in a plastic bag in the patient’s medication storage bin |
|
Discontinuation (or Discharge)
|
Nurses or RTs remove the patient-specific label from the MDI |
| Cleaning # 1 |
Nurses or RTs separate the canister from the plastic mouthpiece, thoroughly wipe the canister and plastic mouthpiece with appropriate disinfection wipes for 2 minutes |
| After thorough MDI drying, nurses or RTs place the canister and mouthpiece back together, place the MDI in a clean plastic bag, and return it to an identified bin in the medication room for pharmacy pick-up |
| Pharmacy reuse assessment |
MDI is returned to a designated area in the pharmacy (spacers are not reused or sent to the pharmacy; MDIs that have not been disinfected are not returned to the pharmacy) |
| Pharmacy staff completes an assessment for re-dispensing (e.g., review of expiration dating, number of doses remaining on counter [MDIs with fewer than 5 doses are not re-dispensed]) |
| Cleaning # 2 |
If MDI is appropriate to re-dispense, pharmacy staff re-cleans the canister and plastic mouthpiece using the same process as above |
| Once cleaned, pharmacy places the canister and plastic mouthpiece back together, seals the MDI in a plastic bag with tamper-evident tape, and applies product labeling |
| Discharge |
MDI used during hospitalization is not sent home with patients |
| If appropriate, patient-specific spacers are sent home with patients |
If a decision is made to move forward with a common MDI canister protocol, we encourage organizations to carefully analyze the process being considered to prevent inadvertent sources of transmission and to emphasize in the protocol the importance of hand hygiene and dual canister disinfection. Organizations should consider excluding patients with presumptive and confirmed COVID-19 infection, or at least segregating common canisters used by presumptive and confirmed COVID-19 patients from those used for the general patient population. ISMP continues to encourage manufacturers to provide smaller “institutional” containers of MDIs to prevent unnecessary waste and lower the cost.